News Center
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
xu@worldbrom.com
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
Lauren@worldbrom.com
shelia@worldbrom.com
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
www.worldbrom.com
Procurement Hotline
0512-52836128Sources:www.worldbrom.com | PublishDate:2026.01.29
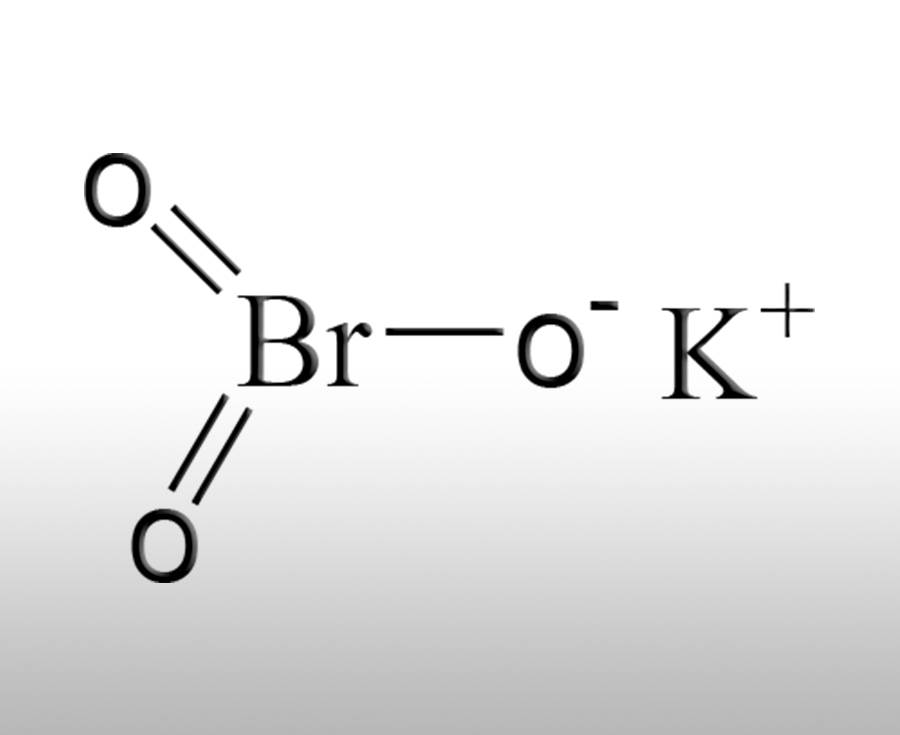
Potassium bromate is an inorganic strong oxidant, which was once used as a flour improver in the food industry. However, due to safety issues, it has been banned in most countries around the world; At present, its applications are mainly concentrated in non food fields such as industrial oxidants, analytical chemistry, and chemical synthesis. Some niche fields also have compliant applications, and all usage scenarios require strict control of its toxicity and oxidizing properties. The following are detailed main uses and usage specifications for each field:
1、 Previous uses in the food industry that have been globally banned (flour improvers)
This is the most well-known use of potassium bromate and its former core application scenario. Due to its clear carcinogenicity, almost all countries such as China, the European Union, the United States, and Japan have banned its use in food.
Principle of action: Potassium bromate has strong oxidizability. During the process of flour fermentation and baking, it can oxidize gluten protein in the flour to form cross-linking between protein molecules, improve the elasticity, toughness and gas retention of gluten, and make the baked bread, Mantou and other flour products larger, more fluffy in texture and softer in taste. At the same time, it can shorten the fermentation time and improve the appearance and processing efficiency of flour products.
Reason for prohibition: Animal experiments have confirmed that potassium bromate has genetic toxicity and carcinogenicity, and cannot be completely decomposed during baking, resulting in residues entering the human body. Long term intake can damage the kidneys and thyroid gland, increasing the risk of cancer; China has officially banned the use of potassium bromate as a food additive since 2005, and any unauthorized addition will face severe punishment.
2、 Current mainstream core applications in the industrial sector
The strong oxidizing ability and stability of potassium bromate are the core advantages of its industrial application. Currently, it is mainly used as an oxidant and additive in chemical, metallurgical, papermaking and other fields. When using it, it is necessary to seal and prevent contact (isolate it from organic matter and reducing agents).
1. Chemical synthesis and oxidants
As an inorganic strong oxidant, it is widely used as an oxidizing agent in various chemical reactions, such as in the synthesis of dyes and pharmaceutical intermediates, oxidizing aromatic ring side chains and sulfur-containing/nitrogen-containing organic compounds; It can also be used as a raw material for preparing other bromides, or as an additive in electroplating solutions in the electroplating industry to enhance the density and Glossiness of coatings.
2. Bleached pulp in the paper industry
Used for pulp bleaching in the paper industry, especially suitable for medium temperature bleaching of wood pulp and straw pulp. Its oxidative properties destroy lignin and pigment molecules in the pulp, improve the whiteness of the pulp, and the bleaching effect is stable. When used in combination with other bleaching agents (such as hypochlorite), it can reduce the amount of bleaching agent used and improve the fiber properties of the pulp; Due to environmental requirements, it has gradually been replaced by milder bromine free bleaching agents, which are only used in little quantities in some specialty paper production.
3. Purification and beneficiation in metallurgical industry
In the metallurgical process of non-ferrous metals such as copper, zinc, and lead, as an oxidative leaching agent, it can oxidize low valent metal oxides in ores into high valent states, enhance the solubility of metals in leaching solutions, and facilitate subsequent extraction and purification; It can also be used as an oxidant in mineral processing to adjust the redox potential of the slurry, optimize flotation efficiency, and improve concentrate recovery rate.
3、 Analytical Chemistry and Laboratory Specific Use
Potassium bromate is a benchmark reagent in analytical chemistry, widely used in titration analysis due to its high purity and good quantification of oxidation reactions. It is a commonly used reagent in physical and chemical testing laboratories.
1. Reference substance for redox titration
As a strong oxidizing reference reagent, it is used to calibrate the concentration of reducing titrants such as sodium thiosulfate and ferrous ammonium sulfate. The titration reaction is correct and the endpoint is obvious. It is suitable for quantitative detection of reducing substances (such as sulfides, sulfites, ferrous ions) in water quality, food, and chemical samples.
2. Core reagents for bromine content method
When used in combination with potassium bromide, bromine (BrO ∝⁻+5Br ⁻+6H ⁺=3Br ₂+3H ₂ O) is generated under acidic conditions. The generated bromine is used for bromine titration to detect phenols, aromatic amines, unsaturated organic compounds, etc. in the sample. It is widely used for component analysis of pharmaceutical, dye, and pesticide samples.
4、 Other niche compliance applications
1. Antibacterial and algicidal agents in the field of water treatment
By utilizing its strong oxidizing properties, it can be used as a bactericidal and algaecide agent for industrial circulating cooling water and swimming pool water, which can quickly kill bacteria, algae, and Microbiota in water, prevent pipeline scaling, and prevent the growth of biofouling; Due to the residual and toxic nature of bromate in water, it is currently only used in little amounts in industrial circulating water (non drinking water) and strictly prohibited in drinking water.
2. Oxidants for fireworks and firecrackers
As an auxiliary oxidant in the production of fireworks and firecrackers, it is used in combination with potassium chlorate and potassium perchlorate to enhance the combustion speed and explosive effect of fireworks, improve the color and brightness of fireworks; Due to its slightly lower oxidizing property than potassium chlorate and higher cost, potassium bromate is only used as a niche additive. When using it, it is necessary to strictly isolate organic matter to prevent spontaneous combustion and explosion.
5、 Core precautions for the use of potassium bromate
Toxicity control: Potassium bromate has acute toxicity (oral LD50 of about 180mg/kg). Inhalation, ingestion, or skin contact can irritate mucous membranes, damage the liver and kidneys, and long-term exposure poses a risk of cancer. Personal protective measures (goggles, corrosion-resistant gloves, gas masks) must be taken in all use scenarios.
Oxidative safety: Potassium bromate is prone to combustion and explosion when exposed to organic matter, reducing agents, flammable materials (such as paper, wood, oil), and when heated or impacted. It should be stored separately in a cool and dry place and strictly prohibited from being mixed with the above substances. During transportation, it should be controlled according to hazardous chemicals.
Environmental requirements: Potassium bromate and its decomposition products (bromides, bromate salts) can cause water pollution. The wastewater and waste residue after use must be treated harmlessly (such as reduction degradation, neutralization and precipitation), and can only be discharged after meeting the standards. It is strictly prohibited to dispose of them at will.