News Center
Popular products
Sales Department Tel:
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
xu@worldbrom.com
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
Lauren@worldbrom.com
shelia@worldbrom.com
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
www.worldbrom.com
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
xu@worldbrom.com
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
Lauren@worldbrom.com
shelia@worldbrom.com
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
www.worldbrom.com
Procurement Hotline
0512-52836128
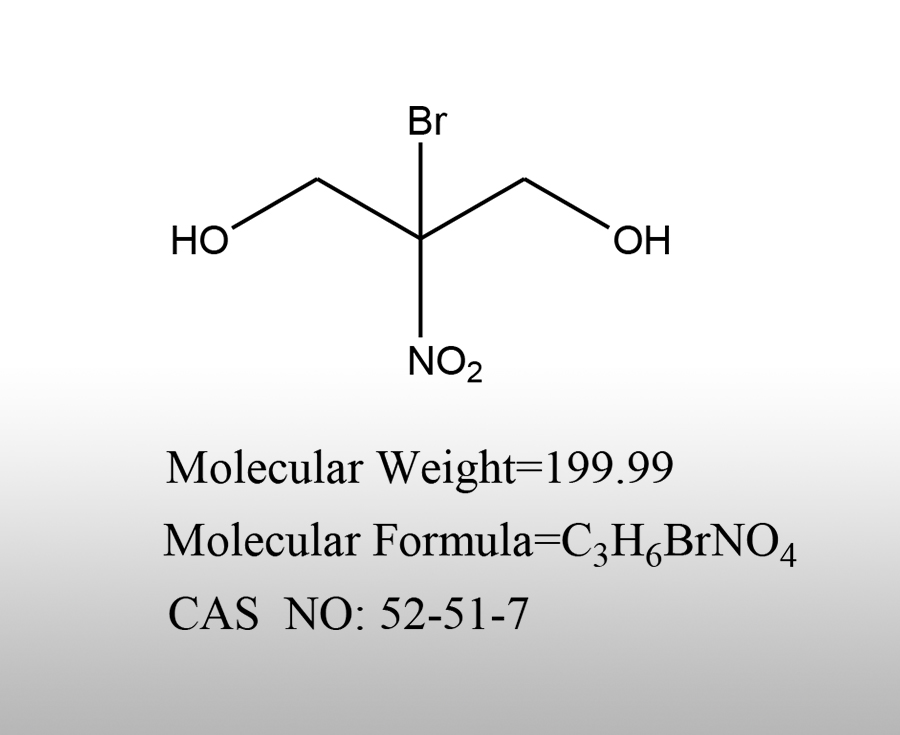
Briefly describe the uses of nitroethanol
Nitroethanol (usually 2-nitroethanol, with the structural formula CH ₂ OHCH ₂ NO ₂) is a type of alcohol compound containing nitro groups, which has certain chemical activity and polarity. Its applications mainly involve organic synthesis, industrial processing, and scientific research fields. The following is a brief overview:Sources:www.worldbrom.com | PublishDate:2025.05.21
1. Organic synthesis intermediates
Purpose:
As a bifunctional compound containing hydroxyl and nitro groups, it is used for the synthesis of pharmaceuticals, pesticides, dyes, and polymer materials.
Example:
Synthesis of nitroalcohol derivatives (such as nitropropanol) for the preparation of antibiotics and anticancer drug intermediates;
Participate in condensation and reduction reactions to generate amines, amino acids, or heterocyclic compounds (such as imidazole and pyridine derivatives).
Features: The electron withdrawing effect of nitro enhances the activity of hydroxyl groups, making it easy to introduce other functional groups.
2. Industrial solvents and additives
Purpose:
As a polar solvent, it dissolves resins, cellulose derivatives (such as nitrocellulose), and some polymer materials, and is used in the coatings and ink industries.
As a co solvent or dispersant, it improves the solubility of polar substances in solvent systems (such as when combined with alcohol and ketone solvents).
Attention: Volatility and toxicity must be controlled, and use is limited to specific industrial scenarios only.
3. Fuel and propellant additives
Potential uses:
The nitro containing structure has a certain amount of energy and can be used as a component of rocket fuel or fuel additives (safety must be strictly controlled).
In research, it is used to improve the combustion efficiency or stability of fuels, but its practical application is limited by its sensitivity and toxicity.
4. Research and laboratory applications
Purpose:
As a chemical reagent, it is used to study the reaction process of nitro compounds, such as nucleophilic substitution and redox reactions.
In materials science, it is used to prepare functionalized polymers containing nitro groups (such as polynitroethanol) and explore their electrical properties or thermal stability.
Safety and Restrictions
Danger: Flammable, toxic, and irritating, strict adherence to hazardous material operating procedures is required.
Regulatory control: Constrained by the Regulations on the Safety Management of Hazardous Chemicals, qualification approval is required for production, storage, and use.
Alternative trend: Due to toxicity and environmental risks, some scenarios have been replaced by low toxicity compounds such as nitropropanediol.