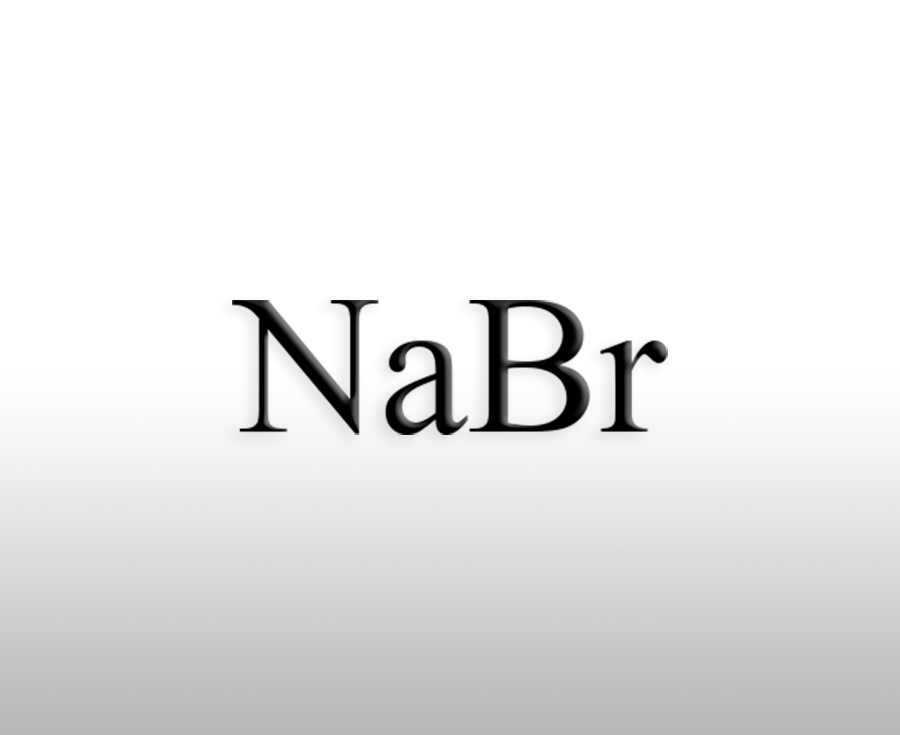News Center
Popular products
Sales Department Tel:
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
xu@worldbrom.com
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
Lauren@worldbrom.com
shelia@worldbrom.com
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
www.worldbrom.com
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
xu@worldbrom.com
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
Lauren@worldbrom.com
shelia@worldbrom.com
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
www.worldbrom.com
Procurement Hotline
0512-52836128
What are the applications of ammonium bromide in the chemical industry
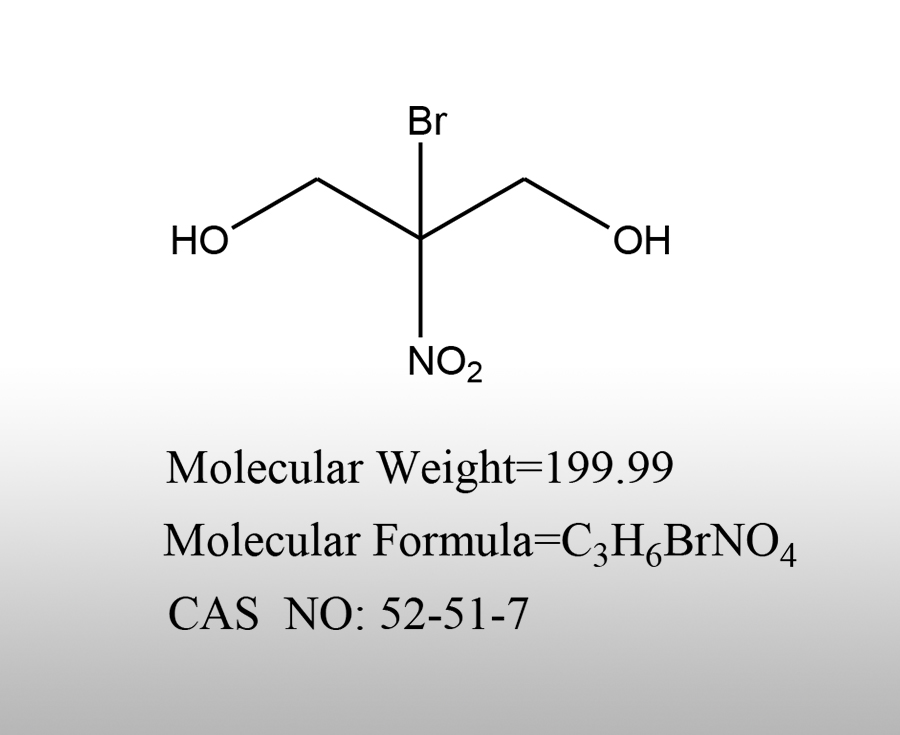
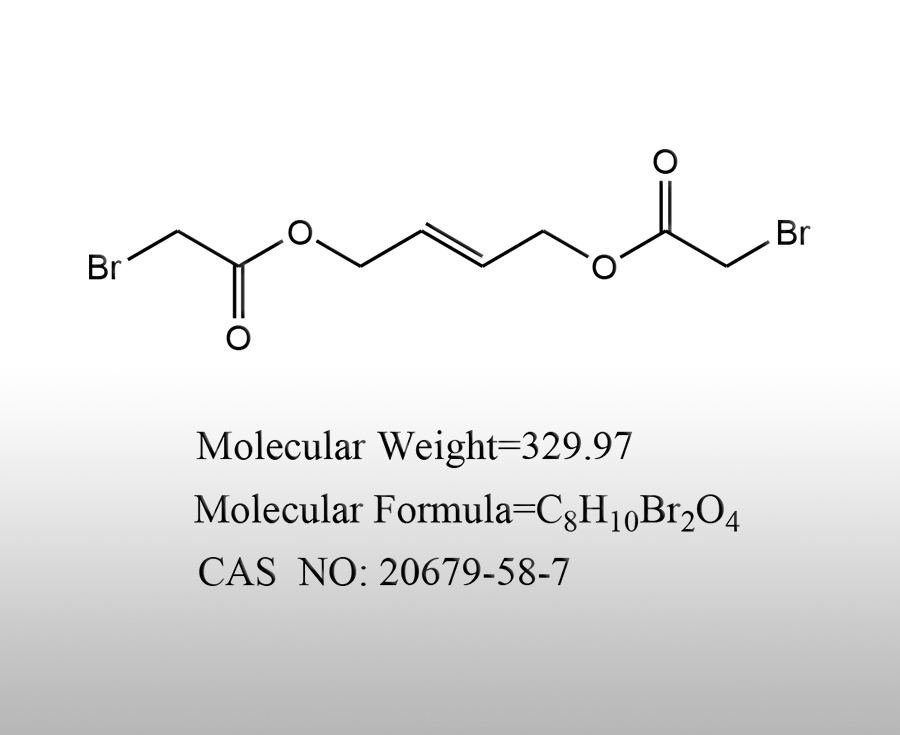
Ammonium bromide (NH ₄ Br) is an inorganic bromide with characteristics such as easy solubility in water and good stability. It is widely used in various fields in the chemical industry due to its chemical properties, as follows:Sources:www.worldbrom.com | PublishDate:2025.07.29
1. Participate in organic synthesis reactions as a bromine source
Ammonium bromide is an important bromide ion donor and is often used as a brominating agent or catalyst in organic chemical synthesis to prepare bromine containing organic compounds
Used for bromination reactions of aromatic compounds, by coordinating with other reagents such as sulfuric acid, hydrogen peroxide, etc., bromine atoms are introduced into the benzene ring or heterocyclic ring to synthesize brominated aromatic hydrocarbons (such as bromobenzene, bromotoluene, etc.), which are important raw materials for dyes and pharmaceutical intermediates.
In some alkylation reactions, it is used as an auxiliary reagent to promote the reaction or to prepare brominated alkanes (such as bromoethane, bromopropane), which can be used as organic solvents or organic synthesis intermediates.
2. Raw materials for preparing other bromides
Ammonium bromide can be converted into other bromide products through processes such as metathesis reactions, and is one of the basic raw materials in chemical production
Reacting with bases such as potassium hydroxide and sodium hydroxide to produce alkali metal bromides such as potassium bromide (KBr) and sodium bromide (NaBr), these products are widely used in fields such as medicine, photography, and analytical reagents.
Reacting with metal oxides or salts to prepare metal bromides such as magnesium bromide (MgBr ₂) and zinc bromide (ZnBr ₂), among which zinc bromide can be used as organic synthesis catalysts, flame retardants, etc.
3. Application of flame retardants in production
Bromine based flame retardants are commonly used high-efficiency flame retardant materials in the chemical industry. Ammonium bromide can be used as a raw material or auxiliary component in the preparation of flame retardants
It is compounded with other compounds containing nitrogen and phosphorus to produce expandable flame retardants. These flame retardants form an expanded carbon layer at high temperatures, isolating heat and oxygen, and are widely used in flame retardant treatment of polymer materials such as plastics and rubber.
Its inherent flame retardant properties can also be used for flame retardant finishing of certain coatings and textiles, achieving flame retardant effects by inhibiting the transfer of free radicals in combustion reactions.
4. Electroplating and metal treatment
In the electroplating industry, ammonium bromide can be used as an additive in electroplating solutions to improve the performance of coatings
Used in the electroplating process of certain metals (such as copper and nickel) to adjust the pH value and ion concentration of the plating solution, promote uniform deposition of metal ions, make the coating denser and brighter, and improve the adhesion and corrosion resistance of the coating.
In metal surface treatment, it can be used as one of the components of etchants for surface etching of precision metal components, removing oxide layers or forming specific patterns.
5. Analytical Chemistry and Reagent Field
As an analytical reagent, it is used in titration experiments or colorimetric analysis in chemical analysis, such as determining silver ion concentration (using silver bromide precipitation reaction), or as a reference material to calibrate instruments.
It is used to prepare buffer solutions, adjust the pH value of the system in some chemical experiments or production processes, and maintain the stability of the reaction environment.
6. Other chemical related applications
In the photosensitive material industry, it can be used as a component of film and photo paper processing solutions, participating in the preparation of photosensitive emulsions or developing and fixing processes, and adjusting the chemical properties of the solution.
Used for preparing certain ionic liquids or electrolyte solutions, as electrolyte components in the field of electrochemistry (such as batteries and capacitors), to improve ion conductivity.