News Center
0512-52836238
0512-52836128
0512-52358728
Sales Department Fax
0512-52836278
Sales Department Email
xu@worldbrom.com
Purchasing Department Tel/ Fax
0512-52836228
Headquarters Address US Office
, No. 18 Haitian Road, Advanced Material Industrial Park, Changshu City, Jiangsu
Tel
+1 832-857-1028
U.S. office mailbox
Lauren@worldbrom.com
shelia@worldbrom.com
U.S. office address
Creekside Park, The Woodlands, Texas 77375, USA
corporate website
www.worldbrom.com
Procurement Hotline
0512-52836128Sources:www.worldbrom.com | PublishDate:2025.09.03
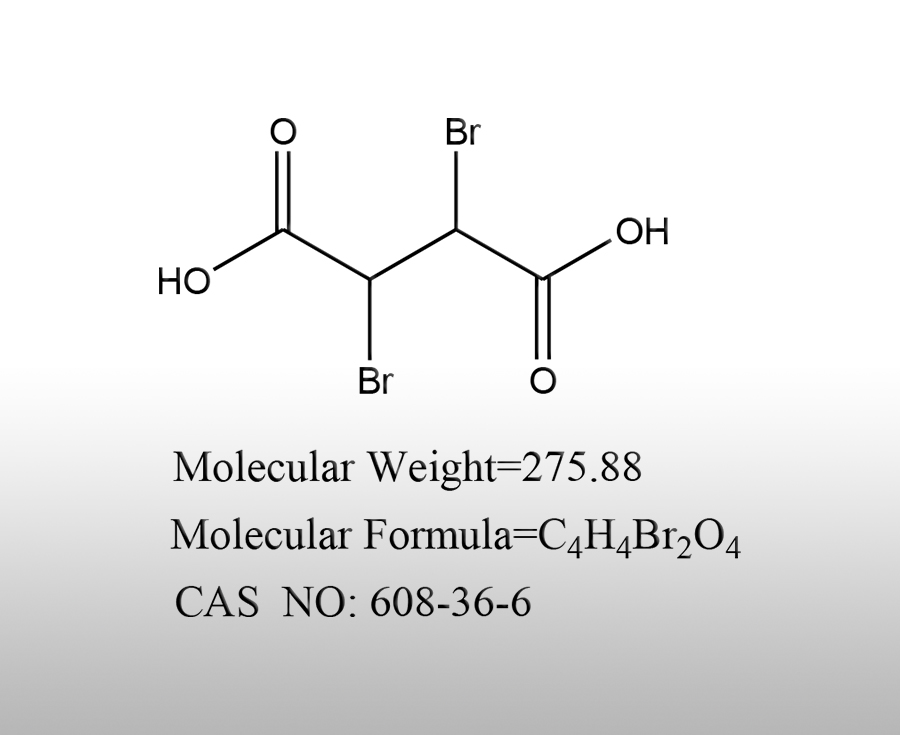
Dibromosuccinic acid (chemical formula C ₄ H ₄ Br ₂ O ₄) is a binary carboxylic acid containing bromine. Due to the presence of two bromine atoms and two carboxyl groups in its molecular structure, and the presence of cis trans isomers (cis dibromosuccinic acid, trans dibromosuccinic acid), its characteristics can be clarified from four dimensions: chemical structure, physical properties, chemical properties, preparation and application:
1. Chemical structure: Significant cis trans isomerization, prominent functional group activity
Characteristics of cis trans isomerization: The core structure is succinic acid (HOOC-CH ₂ - CH ₂ - COOH), where the 2nd and 3rd hydrogen atoms are replaced by bromine atoms. Due to the different relative positions of the two bromine atoms in the double bond (or spatial structure), there are two isomers: cis (2R, 3S) - dibromosuccinic acid, melting point 160-161 ℃) and trans (2S, 3S) - or (2R, 3R) - dibromosuccinic acid, melting point 255-257 ℃). The physical properties (such as melting point and solubility) and chemical activity of the two isomers are significantly different.
Dual functional group structure: The molecule contains two carboxyl groups (- COOH) and two bromine atoms (- Br) simultaneously. The carboxyl group is acidic and can undergo esterification/amidation reactions, while the bromine atom (as a halogenated hydrocarbon functional group) can undergo nucleophilic substitution reactions (such as being replaced by hydroxyl or amino groups), providing a basis for subsequent derivatization.
2. Physical properties: Solid crystal, solubility and isomer related
Appearance and condition: It is a white to light yellow crystalline powder at room temperature, with no obvious odor and good stability (not easily decomposed in a dry environment).
The melting point difference is significant: as mentioned earlier, the cis isomer has a lower melting point (about 160 ℃), while the trans isomer has a significantly higher melting point (about 255 ℃), which is a key physical indicator to distinguish between the two isomers.
Solubility: Easily soluble in polar solvents such as hot water, ethanol, and ether, but difficult to dissolve in cold water and non-polar solvents such as benzene and petroleum ether; The cis isomer usually has slightly higher solubility than the trans isomer due to its stronger molecular polarity.
3. Chemical characteristics: Rich reactivity and directional derivatization
Acidity: Due to the presence of two carboxyl groups, it belongs to a weak binary acid. Water solutions can react with bases (such as NaOH, KOH) to form corresponding disodium salts (such as sodium dibromosuccinate), and can also react with metal oxides to form salts.
Halogenated hydrocarbon reaction: The bromine atom (α - bromine, adjacent to the carboxyl group) in the molecule has high activity and is easily replaced by nucleophiles (such as OH ⁻, NH ⁻, CN ⁻), generating important intermediates such as dihydroxysuccinic acid (tartaric acid derivative) and diaminoduccinic acid.
Elimination reaction: Under the action of strong bases (such as sodium alkoxide), intramolecular elimination reaction can occur, removing two molecules of HBr and generating succinic acid (HOOC-C ≡ C-COOH), which is an important pathway for the synthesis of alkyne carboxylic acids.
Esterification reaction: Carboxyl groups can react with alcohols such as methanol and ethanol to form dibromosuccinic acid esters (such as dimethyl dibromosuccinic acid), which can be used as active intermediates in organic synthesis.
4. Preparation and Application: Controllable synthesis, commonly used for organic intermediates
Mature preparation methods: In industry, maleic acid (maleic acid) or fumaric acid (trans maleic acid) is often used as raw materials, which undergo addition reactions with bromine (Br ₂) in aqueous solution: maleic acid and Br ₂ add to form cis dibromosuccinic acid, and fumaric acid and Br ₂ add to form trans dibromosuccinic acid. The reaction conditions are mild (room temperature, atmospheric pressure), and the products are easily purified by crystallization.
Clear application positioning: mainly used as an organic synthesis intermediate for the preparation of tartaric acid, succinic acid, pharmaceutical intermediates (such as precursors of certain antibiotics and antiviral drugs), as an auxiliary component of flame retardants (due to the presence of bromine, it can enhance the flame retardancy of materials), or as a reagent in the biochemical field (such as crosslinking agent precursors in protein modification research).